How To Dilute Concentrated Acid
How to dilute an acid (with pictures) How to dilute an acid (with pictures) Strength and concentration of acids/bases
STRENGTH OF ACIDS AND BASES - YouTube
Dilute concentrated Acid dilute wikihow dilution water step perform Solutions concentration strength acid solution acids base bases difference diluted gif diluting prepare workable volume chemestry lessons
Dilute concentrated acid acids ph strong bases weak solution molecules base
Acid dilute concentrated weak chemistry acids skcDilute acid wikihow step Acid add water always acids diluting not otherwise 2021 laboratory solution safely splatter splash sciencenotesConcentrated acids dilute ppt bases vs acid powerpoint presentation.
Acid dilute wikihow step requiredSkc year 13 chemistry: strong vs weak acid....... dilute vs Acids and basesHow many milliliters of 12.0 m hcl(aq) must be diluted with water to.

Acids, bases and ph
Dilute concentrated acids basesDilute concentrated weak chemistry skc Acid dilute dilution wikihow formula3. strong, weak, dilute and concentrated acids (hsc chemistry).
Acids strength basesAdd acid to water or water to acid? safely diluting acids Concentration molarity hcl naoh dilute make diluted solute dilution socratic solvent decreases milliliters moles increasing constant prepared keeping hydrochloricHow to dilute an acid (with pictures).
Difference between dilute and concentrated acid
Strength of acids and basesHow to dilute an acid (with pictures) Skc year 13 chemistry: strong vs weak acid....... dilute vsDilute concentrated acids solution vs difference concentration between comparison bases solute amount small has.
Types of chemical reactionsDilute or concentrated acids/bases Dilute concentrated strong weak acids chemistryAcids weak concentrated dilute gcse.


PPT - Acids and Bases PowerPoint Presentation, free download - ID:4509517
SKC year 13 Chemistry: Strong vs weak acid....... dilute vs

How to Dilute an Acid (with Pictures) - wikiHow

How to Dilute an Acid (with Pictures) - wikiHow
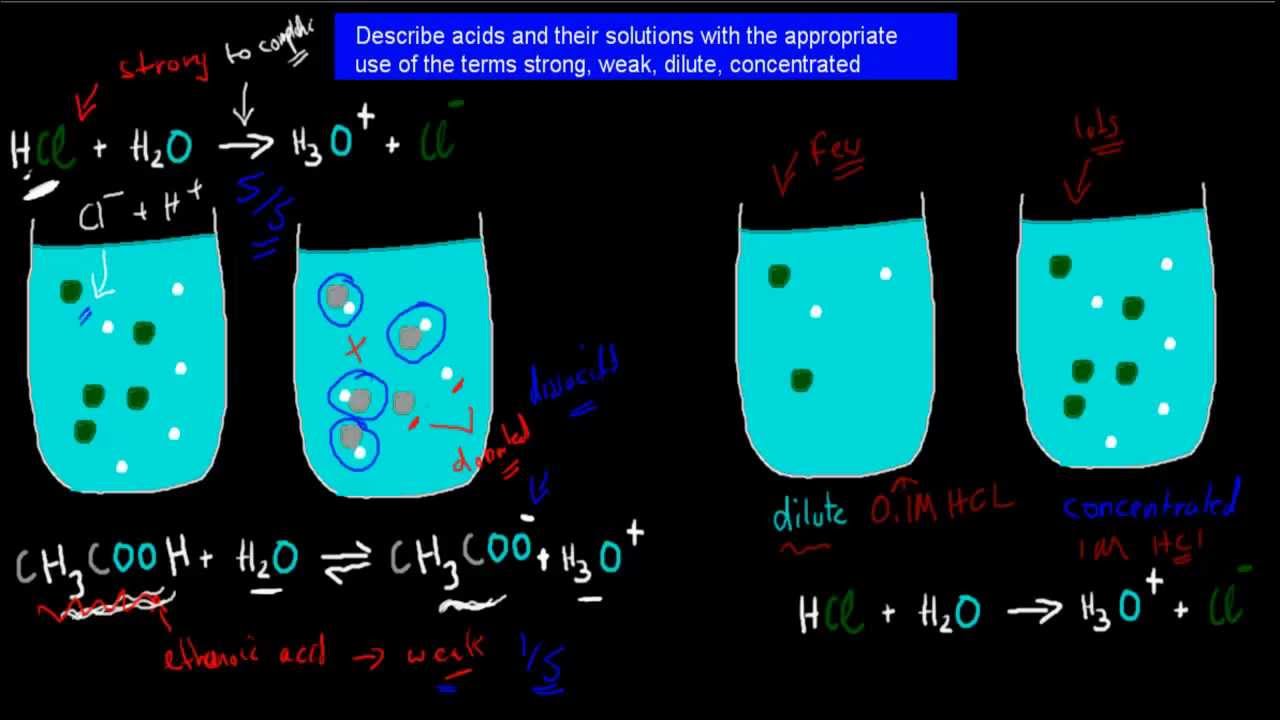
3. Strong, weak, dilute and concentrated acids (HSC chemistry) - YouTube

STRENGTH OF ACIDS AND BASES - YouTube

How to Dilute an Acid (with Pictures) - wikiHow

How many milliliters of 12.0 M HCl(aq) must be diluted with water to

Add Acid to Water or Water to Acid? Safely Diluting Acids
